Kyverna Therapeutics Submits IND for Novel CAR T-Cell Therapy to Treat Lupus Nephritis
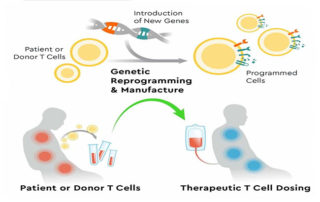
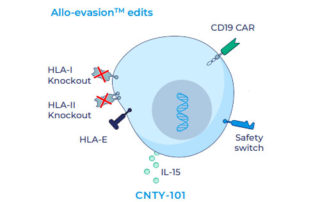
webmaster2022-10-26T14:30:28+00:00Company files its first Investigational New Drug application for its lead program KYV-101, a novel fully human CD19 CAR T-cell therapy, for the treatment of lupus nephritis. Kyverna’s therapeutic platform combines advanced T-cell engineering and synthetic biology technologies to suppress and eliminate...