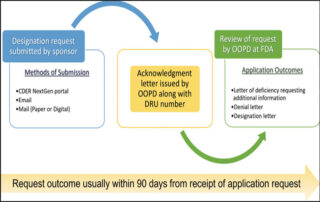
New preclinical data from Sana Biotechnology highlights the potential of CAR T cell therapy
webmaster2024-07-10T19:48:37+00:00Preclinical data demonstrates that hypoimmune-modified allogeneic iPSCs evade immune response and rejection without immunosuppression in a non-human primate model and long-term survival and immune evasion was at least equivalent to the survival of autologous iPSCs...