News
Your source for the latest updates, breakthroughs, and insights in drug development and manufacturing. Explore our featured stories, press releases, and event announcements to stay informed about our innovations and contributions to drug development and manufacturing advancements.
Biodistribution testing of cell and gene therapies
Cell and gene therapy drug products represent a rapidly growing field in the development of new treatments for a wide range of diseases. These therapies have the potential to revolutionize medicine by offering cures rather than just managing symptoms. However, with this potential comes unique challenges, particularly with regard to biodistribution testing...
Gene editing of human stem cells to treat rare disease
Gene editing has the potential to transform the lives of patients with previously untreatable conditions, offering hope for a better future. As the technology continues to advance, it is likely that we will see more breakthroughs in this field in the coming years, bringing us closer to a world where rare diseases are...
Avance Biosciences™ licenses SeQure Dx’s GUIDE-seq off-target NGS analysis technology
Avance Biosciences™ (Avance), a leading CRO that provides GLP/CGMP-compliant biological testing services that aid drug development and manufacturing, announced today that it has signed a licensing agreement with SeQure Dx, a cutting-edge gene-editing diagnostics company...
Quality Assurance of E. coli Cell Banks used in Biopharmaceutical Production
The use of E. coli cell banks poses some challenges and requires careful attention to quality control and quality assurance to ensure that the cells are of the highest quality and free from contamination or genetic mutations. Testing may include identity, purity, viability, characterization, and stability testing...
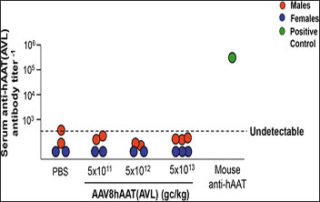
Weill Cornell study demonstrates safety of intravenous administration of an AAV8 gene therapy
Researchers report on the safety of a gene therapy to treat the common autosomal recessive hereditary disorder alpha 1-antitrypsin (AAT) deficiency in a new article in the peer-reviewed journal Human Gene Therapy. In ATT deficiency, neutrophil proteases destroy the lung parenchyma, the portion of the lungs...
FDA clears Sana Biotechnology’s CAR T Therapy IND application for patients with B-cell malignancies
The goal of the hypoimmune platform is to overcome the immunologic rejection of allogeneic cells, which if true for SC291 may result in longer CAR T cell persistence and a higher rate of durable complete responses for patients with B-cell lymphomas or leukemias. The hypoimmune platform includes disruption of major...