News
Your source for the latest updates, breakthroughs, and insights in drug development and manufacturing. Explore our featured stories, press releases, and event announcements to stay informed about our innovations and contributions to drug development and manufacturing advancements.
NGS for ID and characterization of AAV genomes
Adeno-associated virus (AAV) is a small, non-pathogenic, and non-enveloped virus that has become an important tool for gene therapy research and applications. AAV is a type of virus that requires a helper virus (such as adenovirus) to replicate, and it has a high degree of stability and safety, as it does not...
Quality control for gene therapies
Strict quality requirements for gene therapies are designed to ensure the safety, efficacy, and consistency of these innovative treatments, and to minimize the risks associated with their use. These requirements are necessary to provide confidence to regulatory agencies, healthcare providers, and patients that gene therapies are safe and effective.
Potency testing for cellular and gene therapies
Potency testing is an essential part of developing cellular and gene therapy (CGT) products. The US Food and Drug Administration (FDA) requires sponsors for Investigational New Drug (IND) and Biologics License Application (BLA) to provide in vitro or in vivo data to demonstrate the potency of the drug product...
Protein expression biodistribution studies
Protein expression analysis is a critical process for researchers in the life sciences industry. The ability to accurately and reliably measure protein expression and distribution is essential for understanding the underlying biological mechanisms of disease and for developing effective therapeutic interventions...
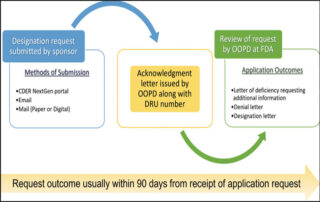
Gene therapy developers can benefit from orphan drug and rare pediatric disease designations
Providing an overview of the submissions process and examples of U.S. Food and Drug Administration (FDA) applications for Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD), a new article can help developers of gene therapies for rare genetic diseases...
NICE recommends life-changing gene therapy for children with ultra-rare genetic disorder
A single dose gene therapy designed to correct the underlying genetic defect, eladocagene exuparvovec (also called Upstaza and made by PTC Therapeutics), is infused directly into the brain through a minimally invasive procedure – the first gene therapy to be administered in this way...