News
Your source for the latest updates, breakthroughs, and insights in drug development and manufacturing. Explore our featured stories, press releases, and event announcements to stay informed about our innovations and contributions to drug development and manufacturing advancements.
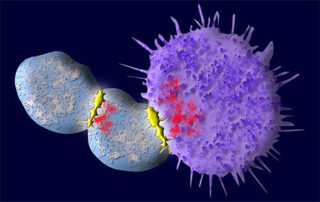
Study reveals new way to reduce friendly fire in cell therapy
In a promising form of immunotherapy known as CAR T-cell (chimeric antigen receptor) therapy, the patient’s T cells are engineered to better recognize and attack antigens on the surface of cancer cells. In treatments currently approved for use in battling lymphoma and leukemia...
FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes
The U.S. Food and Drug Administration approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment of type 1 diabetes. Lantidra is approved for the treatment of adults with type 1 diabetes who are unable to approach target glycated hemoglobin (average blood glucose levels) because of current...
Enhancing Safety and Efficacy – Biodistribution Services for Advanced Cell and Gene Therapies
As the field of advanced cell and gene therapies continues to grow exponentially, ensuring their safety and efficacy becomes paramount. Biodistribution studies, which evaluate the distribution of viral vectors and transgenes in the body, play a crucial role in pre-clinical pharmacokinetics and toxicity assessments...
A historic first for gene therapy
Sarepta Therapeutics announces FDA approval of ELEVIDYS, the first gene therapy to treat Duchenne Muscular Dystrophy. ELEVIDYS addresses the root genetic cause of Duchenne – mutations in the dystrophin gene that result in the lack of dystrophin protein – by delivering a gene that codes for a shortened form of dystrophin to muscle cells known as ELEVIDYS micro-dystrophin...
2seventy bio presents broad range of new data highlighting novel approaches across its cell therapies portfolio
2seventy bio announced new data featuring novel approaches combining the company’s platform CAR T cell and T cell receptor technology and unique cell therapy engineering capabilities to potentially enhance treatment potency in a range of cancers...
New preclinical data from Sana Biotechnology highlights the potential of CAR T cell therapy
Preclinical data demonstrates that hypoimmune-modified allogeneic iPSCs evade immune response and rejection without immunosuppression in a non-human primate model and long-term survival and immune evasion was at least equivalent to the survival of autologous iPSCs...