News
Your source for the latest updates, breakthroughs, and insights in drug development and manufacturing. Explore our featured stories, press releases, and event announcements to stay informed about our innovations and contributions to drug development and manufacturing advancements.
Gene therapy biotech LEXEO Therapeutics files for IPO
LEXEO Therapeutics will use the net proceeds from this offering to advance the clinical development of cardiac and neurological disease gene therapies. LEXEO Therapeutics, in its SEC filing, said it intends to use the net proceeds from this offering, together with their existing cash, to advance the clinical development of LX2006, LX2020 and LX1001...
FDA takes action on updated mRNA COVID-19 vaccines
The mRNA COVID-19 vaccines approved and authorized today are supported by the FDA’s evaluation of manufacturing data to support the change to the 2023-2024 formula and non-clinical immune response data on the updated formulations including the XBB.1.5 component...
FDA issues new draft guidelines on cell and gene therapy manufacturing changes
Specifically, the guidance recommends conducting analytical comparability studies to provide scientific evidence of the impact that manufacturing changes may have on the safety, potency, and purity of human cellular and gene therapy products...
First IND clearance for an allogeneic, off-the-shelf NK or CAR-T cell therapy candidate in autoimmune disease
Artiva Biotherapeutics announced today that the U.S. Food and Drug Administration has cleared the company’s Investigational New Drug application for AlloNK®, in combination with rituximab for treatment of systemic lupus erythematosus in patients with active lupus nephritis...
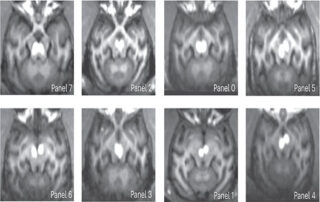
Study shows promise of gene therapy for alcohol use disorder
A form of gene therapy currently used to treat Parkinson’s disease may dramatically reduce alcohol use among chronic heavy drinkers, researchers at Oregon Health & Science University and institutions across the country have found...
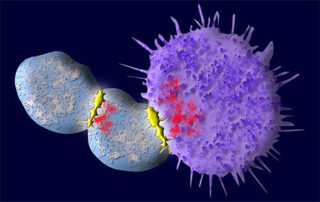
Study reveals new way to reduce friendly fire in cell therapy
In a promising form of immunotherapy known as CAR T-cell (chimeric antigen receptor) therapy, the patient’s T cells are engineered to better recognize and attack antigens on the surface of cancer cells. In treatments currently approved for use in battling lymphoma and leukemia...