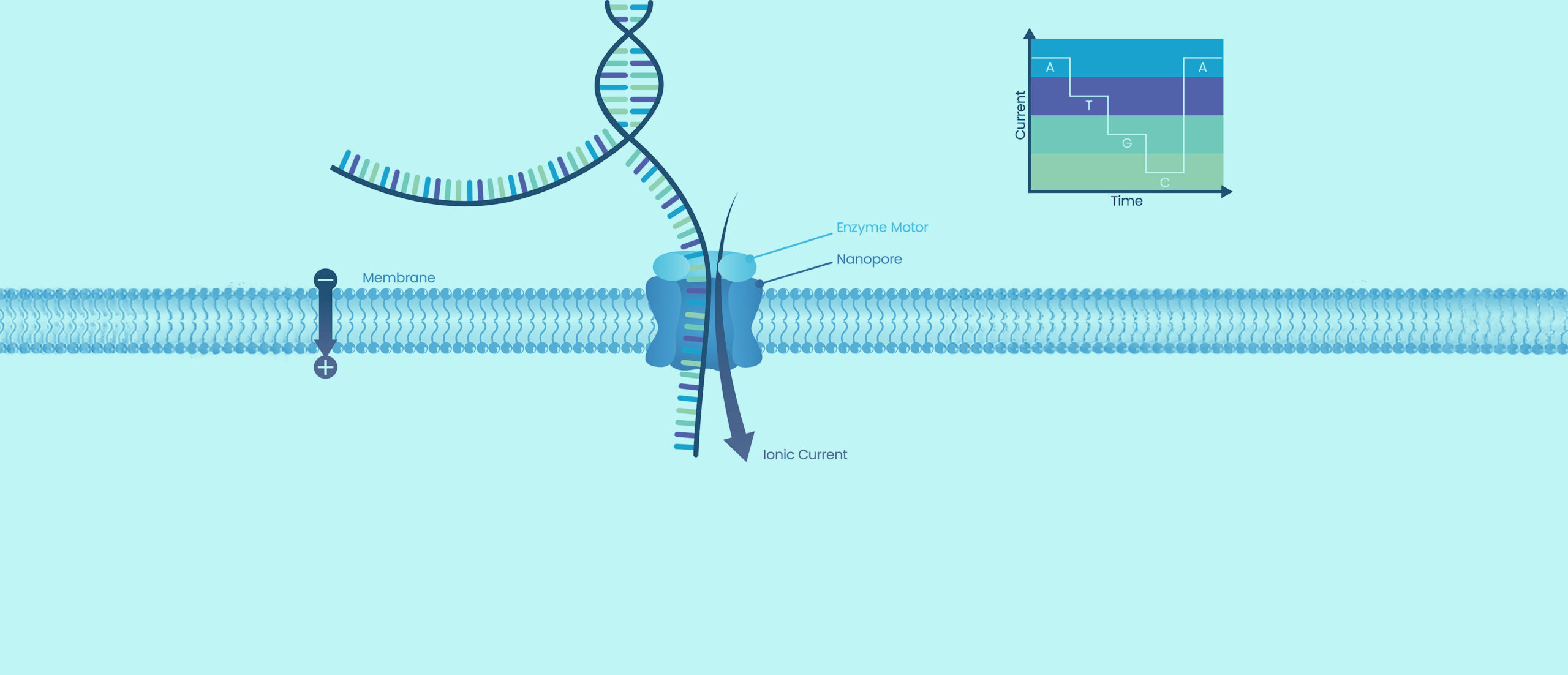
Nanopore Sequencing Services
Nanopore sequencing has seen its applications in the development and manufacturing of biological drugs and therapies expand in recent years. This technology aids in gene integration site studies, elucidates complex genomic sequences with tandem repeats, and facilitates quality control of RNA products through direct sequencing, to name a few. Its long-read sequencing capabilities allow for detailed characterization of complex genetic structures and modifications, ensuring the integrity and stability of therapeutic products.
Our Expertise
Avance Biosciences stands as a global leader in sequencing services with a rich legacy of experience. Pioneering the adoption of Illumina sequencing into our GMP lab in 2013, we now proudly introduce Nanopore platforms while adhering strictly to GMP and GLP regulations. Leveraging our deep understanding of Nanopore sequencing chemistry, we are uniquely positioned to assist our clients in developing innovative solutions tailored to their analytical and bioanalytical requirements.
Nanopore Sequencing Services
Integration Site Analysis
Nanopore sequencing enables precise identification and characterization of gene integration sites, particularly in gene-edited cells of clonal origin, such as CHO cell banks.
Complex DNA Sequence Analysis
Nanopore long-read sequencing excels in traversing repetitive sequences, providing a more comprehensive view of genomic architecture compared to Illumina NGS.
Complex RNA Sequence Analysis
Sequencing synthetic RNA directly on Nanopore platforms eliminates the need for PCR amplification, thereby enhancing the reliability of RNA identity and integrity tests.
Key Features of our Services
At Avance Biosciences, we harness cutting-edge Nanopore sequencing technology to empower our clients in advancing new biologics and cell and gene therapies. Our services are meticulously crafted to not only meet but exceed the exacting scientific and regulatory standards expected by both our clients and regulatory agencies.
- Custom Assay Design: Our NGS labs excel in developing and validating bespoke assays tailored to fulfill the distinctive testing requirements of our clients.
- New Assay Implementation: Our NGS scientific team, drawing upon their in-depth knowledge of sequencing chemistries, has a demonstrated track record of seamlessly implementing new assays from literature to the QC lab.
- Regulatory Compliance: We have implemented a variety of measures to ensure compliance with FDA GMP, GLP, and Part 11 regulations. These measures include operational controls and database-driven audit trails, which guarantee robust adherence to FDA regulations.